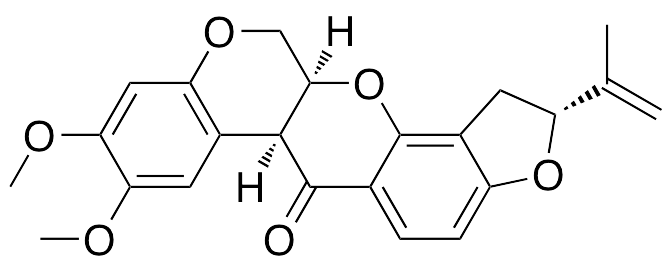
Description
Rotenone is a pesticide that exhibits genotoxic, pro-oxidative, and neurodegenerative properties. In vitro, rotenone induces formation of micronuclei and modifies the microtubule organizing centers of mitotic spindles. In other cellular models, rotenone inhibits the mitochondrial electron transport complex I, altering mitochondrial respiration and inducing mitochondrial oxidative stress. Rotenone also inhibits background K+ currents, causing rapid cell membrane depolarization and increases in intracellular Ca2+. In vitro, this compound also activates microglial superoxide release, resulting in neurodegeneration of dopaminergic neurons, mimicking pathologies associated with Parkinson’s disease.