Description
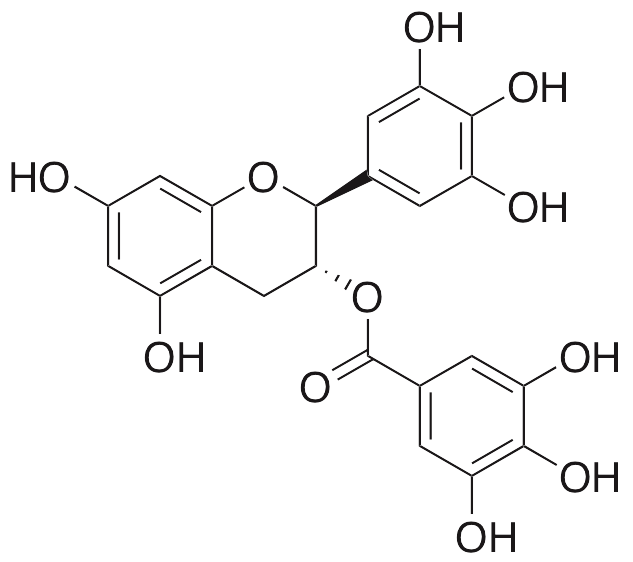
Gallocatechin galleate (GCG) is a polyphenol originally derived from a variety of sources, including green tea, coffee, safflower, and almonds. GCG exhibits a wide variety of beneficial properties, including anti-diabetic, anti-osteoporotic, antiviral, anti-parasitic, and anticancer chemotherapeutic activities. GCG inhibits amyloid formation by islet amyloid polypeptide (amylin) and also inhibits glutathione-S-transferase in cellular models. GCG inhibits HIV integrase, preventing its ability to bind to DNA. Separately, this compound prevents hatching of nematodes in vivo. GCG also has positive effects on bone metabolism, decreasing osteoclastogenesis and inhibiting osteoclast differentiation.