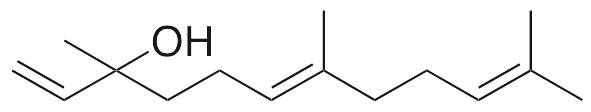
Description
Nerolidol exhibits neuromodulatory, sedative, antibacterial, antifungal, antioxidative, and anticancer activities. Nerolidol inhibits acetylcholinesterase (AChE) and acts as a sedative in vivo in the open field test. This compound also displays antimicrobial activity against gram negative and gram positive bacteria as well as fungi. In vivo, nerolidol increases superoxide dismutase and catalase activity and decreases lipid peroxidation and nitrite levels in the hippocampus. Additionally, nerolidol modulates F0F1-ATP synthase, decreasing mitochondrial transmembrane electric potential, inhibiting cell growth, and causing death in hepatocarcinoma cells.