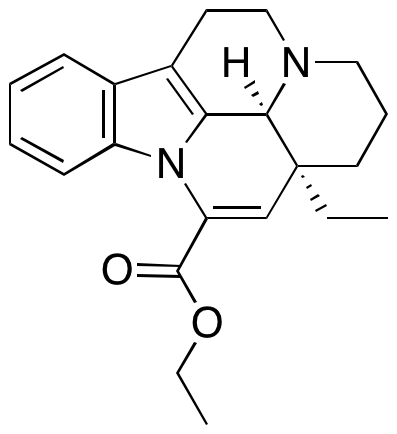
Description
Vinpocetine is a synthetic analogue of the alkaloid apovincamine, which is isolated from the lesser periwinkle plant. It has been shown to inhibit lipopolysaccharide-induced lung inflammation in mice by targeting nuclear factor kappa B activation. Furthermore, it is known to be a safe nootropic agent and is commonly used to enhance memory and to treat various neurological diseases.