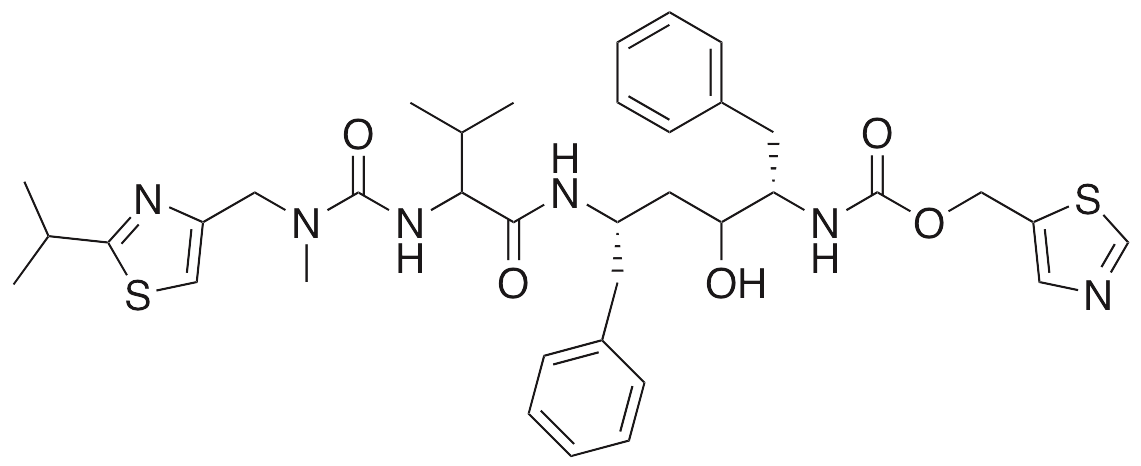
Description
Ritonavir is an HIV protease inhibitor that is commonly used as a component of highly active anti-retroviral therapy (HAART) in the treatment of HIV infection. Ritonavir exhibits antiviral, anti-angiogenic, neuroprotective, and hyperlipidemic activities. Ritonavir inhibits expression of VEGF and HIF-1α, decreasing proliferation in retinal epithelial cells and indicating potential use as a treatment for ocular diseases. Ritonavir also inhibits translocation of apoptosis-inducing factor (AIF), activates caspase 9, and inhibits permeability alterations in the mitochondrial membrane potential, preventing apoptosis in retinal photoreceptor cells and macrophages. This compound decreases levels of sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) and intracellular Ca2+, increasing endoplasmic reticular stress and injury. Additionally, ritonavir increases levels of IL-6 and decreases levels of adiponectin, GLUT4, and fatty acid synthase, inhibiting lipogenesis and increasing lipodystrophy; this compound also increases levels of VLDL.