Description
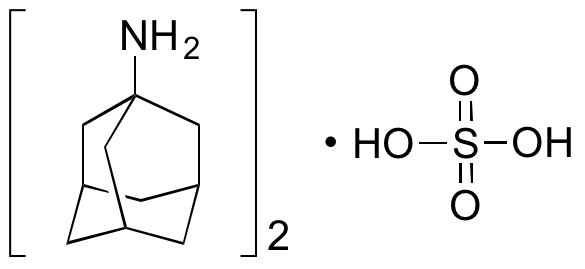
Amantadine exhibits neuroprotective and antiviral activity. Amantadine directly inhibits the viral M2 proton channel. This compound is also used to treat Parkinson’s disease; it inhibits monoamine oxidase A (MAO-A), α7 nicotinic acetylcholine receptors (nAChRs), norepinephrine transporters (NET), and NMDA receptors (through acceleration of channel closure).