Description
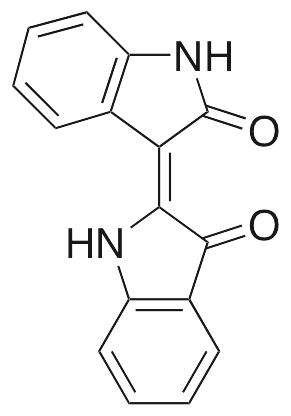
Indirubin is a bisindole isomer of indigo found in Indigo naturalis. It exhibits anti-inflammatory, anti-angiogenic, and anticancer chemotherapeutic activities. In vivo, indirubin decreases levels of IgE and production of inflammatory cytokines, decreasing overall inflammation as well as skin lesion thickness and hyperkeratosis. In vitro, it downregulates expression of CDC25B and inhibits EGFR and CDKs. In leukemia cells, this compound inhibits expression of IAP1, IAP2, Bcl-2, Bcl-xL, TRAF1, cyclin D1, c-Myc, COX-2, and MMP-9. In endothelial cells, it inhibits cell migration, tube formation, and survival; additionally, it suppresses VEGFR2-mediated JAK/STAT signaling. Indirubin also inhibits growth of prostate tumors in animal models.