Description
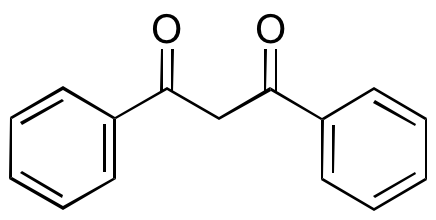
Dibenzoylmethane (DBM) is an aromatic 1,3-diketone acetylacetone derivative that is originally found in Glycyrrhiza (licorice). DBM derivatives are very photostable and are often used as sunscreen components; they are also cytoprotective. DBM itself exhibits anti-inflammatory, cytoprotective, anticancer chemotherapeutic, and chemopreventive activities. DBM induces G2/M phase cell cycle arrest and downregulates expression of phospho-rb, cyclin D1, and cyclin A, inhibiting cell proliferation and tumor growth in prostate cancer models; this compound also inhibits the development of intestinal adenoma in vivo. In other models, DBM activates Nrf2-dependent phase II enzymes, inhibiting DNA adduct formation; it also prevents TNF-induced activation of NF-κB.